
#Iso 17025 2017 changes free#
provides model of a quality system that is natural simple and free from excessive paperworkįor any questions, don't hesitate to email us: click here.
#Iso 17025 2017 changes iso#
defines a baseline system that satisfies ISO 17025 requirements document titles and numbers exactly match the standard
#Iso 17025 2017 changes verification#
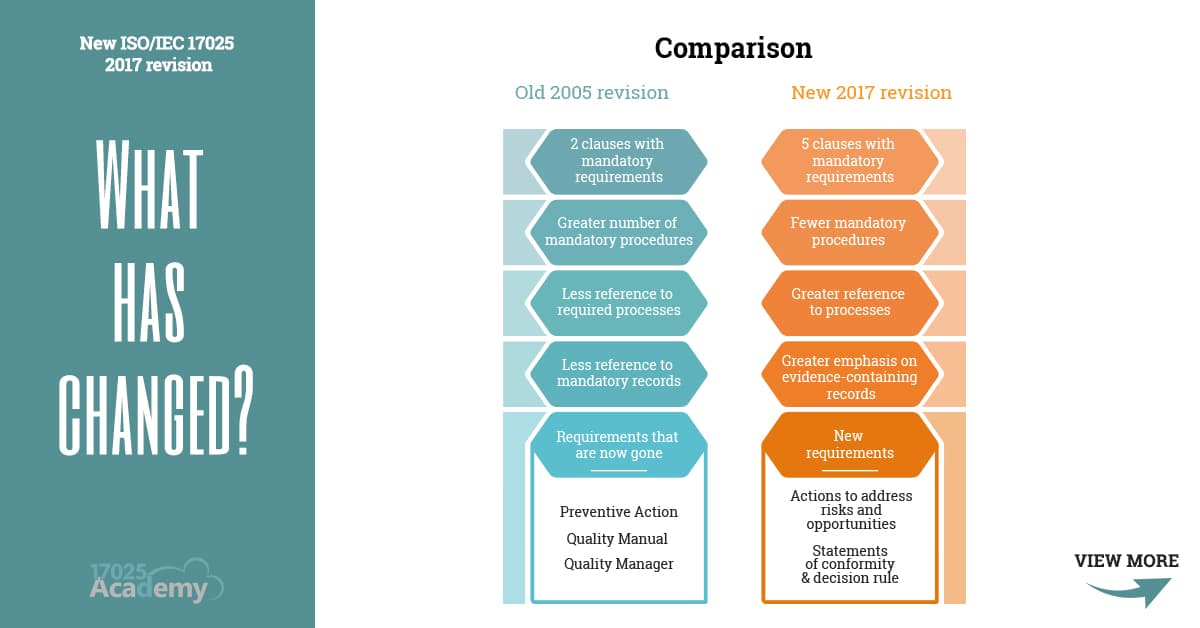
It is specifying the basic requirements for the competence verification of laboratories carrying out testing and calibration activities, focused in meeting customer expectations and keeping organised laboratory records and documents (ISO/IEC 17025, 2017). covers all sections and subsections of the ISO 17025 standard ISO/IEC 17025 is the global quality standard for testing and calibration laboratories. Read more about the changes between ISO/IEC 20. There are structural changes in the document, but the technical changes do not seem to be large, and most simply clarify existing requirements. ISO/IEC 17025 has had two revisions since then, it was revised in 2005 and then in 2017 to keep up-to-date with the industry, introducing technology into the requirements, and also trying to follow the ISO 9001:2015 standard as closely as possible. Greater flexibility in the guidelines for processes, procedures, documented information, and. The employment of this information has enabled some reduction of. Test reports and certificates can be accepted from one country to another without the need for further testing, which, in turn, improves international trade. contains a quality manual, procedures and quality records that comply with ISO 17025 In summary, 17025:2017 differs much less from 17025:2005 than it appears at first sight. As for meeting the current industry needs, the changes to ISO/IEC 17025:2017 include: A new chapter on risk-based thinking has been added. ISO/IEC 17025:2017 also helps facilitate cooperation between laboratories and other bodies by generating wider acceptance of results between countries. Our package will help you implement your quality system at a fraction of the cost and time you We provide ISO 17025 implementation packages which help in writing the required documentation and assist with implementation of the accreditation requirements.įor the introduction of the ISO 17025 standard, you need: It is also the basis for accreditation from an accreditation body.Ī prerequisite for a laboratory to become accredited is to have a documented quality system. Laboratories use ISO 17025 to implement a quality system aimed at improving their ability to consistently produce valid results.
The need to gain ISO 17025 compliance and accreditation impacts many laboratories.

As for meeting the current industry needs, the changes to ISO/IEC 17025:2017 include: A new chapter on risk-based thinking has been added. The above changes to the international standard are focused on trends with ISO documents. The current release was published in 2017. Changes to the Calibration Laboratories Standard. ISO 17025 is a quality standard for testing and calibration laboratories. There is a new emphasis on 'risks and opportunities', clearer reference.

Among other things, the new Standard has a substantially revised structure, including different management system options. ISO 17025: 2017 Quality manual, procedures, templates, examples 2017 saw the publication of a new version of ISO/IEC 17025 - General requirements for the competence of testing and calibration laboratories.


 0 kommentar(er)
0 kommentar(er)
